A mathematical model developed by researchers from the University of Birmingham, Queen Mary University of London, and the Centre de Recerca Matemàtica reveals how chromaffin cells adapt to the loss of SDH-b—a key metabolic enzyme subunit whose dysfunction is linked to pheochromocytomas. By simulating mitochondrial dynamics, the model predicts hallmark features of pseudohypoxia and provides valuable insights into cellular resilience and potential therapeutic targets.
A Computational Lens on Cellular Resilience
In the study A Mathematical Exploration of SDH-b Loss in Chromaffin Cells, published in the Bulletin of Mathematical Biology, researchers Elías Vera-Sigüenza, Himani Rana, Fabian Spill, and Daniel A. Tennant (University of Birmingham), Ramin Nashebi and Katarína Kl’uvčková (Queen Mary University of London), and Ielyaas Cloete (Centre de Recerca Matemàtica) investigate how the loss of a key metabolic enzyme subunit, SDH-b, affects chromaffin cells—specialized endocrine cells located in the adrenal medulla. Through a detailed computational model, the authors reveal how these cells adapt to mitochondrial dysfunction, offering new insights into the metabolic reprogramming associated with rare tumors such as pheochromocytomas, and contributing to a broader understanding of cellular resilience under metabolic stress.
“This study was conceptualised by Elías Vera-Sigüenza, Katarína Kl’učková and Daniel Tennant.
My motivation comes from a deep interest in studying aspects of metabolism, especially calcium signalling involved in cellular metabolism and calcium regulates many of the metabolic fluxes under consideration in this study.
However, this study was motivated by the fact that paraganglioma and phaeochromocytoma is an extremely rare disease with unmet need, and these pathologies have similar metabolic signatures to ageing heart, so we could leverage many insights from a well-studied topic to gain insights into a poorly studied and understood disease.”
— Ielyaas Cloete (CRM)
The Role of SDH in Cellular Metabolism
Succinate dehydrogenase (SDH) is a central enzyme in cellular metabolism, linking the tricarboxylic acid (TCA) cycle to the mitochondrial electron transport chain (ETC). The loss of its SDH-b subunit initiates a cascade of metabolic and signaling disruptions that alter how chromaffin cells produce and manage energy. This disruption leads the cells into a state that mimics oxygen deprivation, even when oxygen is present—a phenomenon known as pseudohypoxia. This metabolic reprogramming is a hallmark of phaeochromocytomas, rare tumors that originate from these adrenal cells.
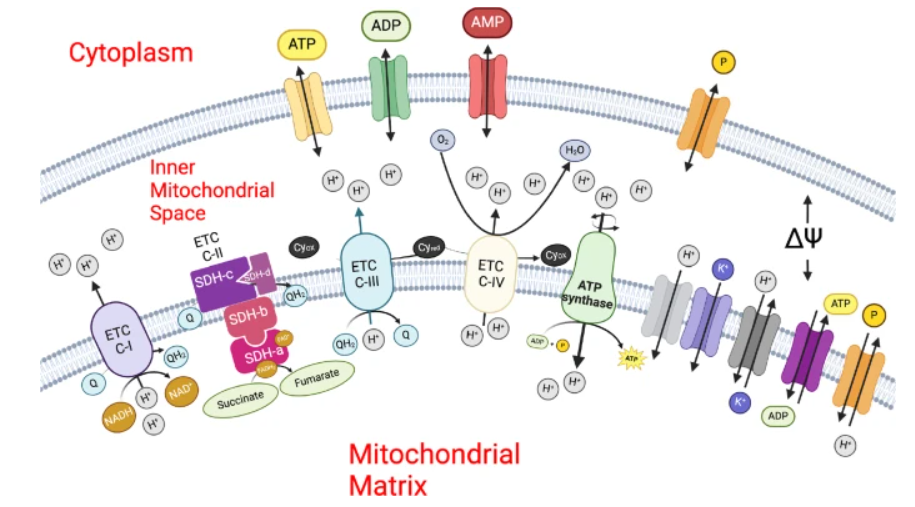
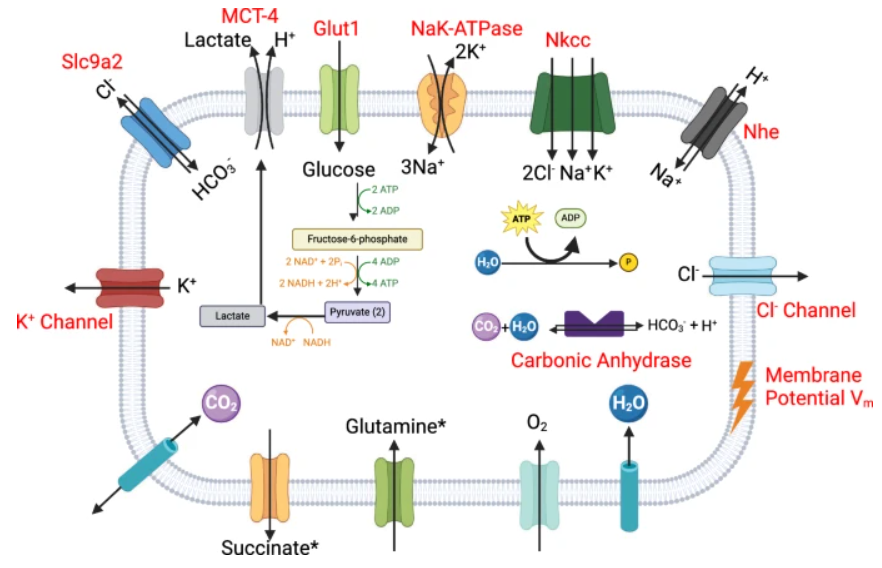
Schematic diagram of the inner mitochondrial membrane space reaction fluxes of the model associated with the electron transport chain model. Figure taken from the original publication.
Modeling the Cellular Response
To investigate how chromaffin cells adapt to this dysfunction, the researchers developed a mathematical model that simulates key aspects of cellular metabolism. It tracks changes in metabolite concentrations, ion fluxes, membrane potentials, and compartmental volumes across four interconnected regions of the cell.
The model incorporates mechanisms for ion transport and pH regulation, as well as control of osmotic volume and mitochondrial swelling. It also applies an electrical circuit-based approach to simulate membrane potential using Kirchhoff’s law. To ensure computational efficiency, glycolysis is represented as a simplified net reaction, allowing the model to focus on mitochondrial dynamics.
Subscribe for more CRM News
Experimental Validation and Key Predictions
Validation of the model was carried out through experimental techniques such as glucose labeling, metabolic tracing, and quantification of glucose, lactate, sodium, and total protein. These experiments confirmed several of the model’s predictions, including the following key metabolic and structural changes:
- SDH-b knockout cells showed increased lactate and reduced pyruvate and glucose levels, indicating a shift toward glycolysis and a pseudohypoxic phenotype.
- Mitochondrial pyruvate levels were reduced, suggesting impaired import and a shift to cytosolic metabolism.
- Despite the energetic cost imposed by the reversal of ATP synthase activity—where ATP is hydrolyzed to maintain mitochondrial membrane potential—the model shows that intracellular ATP levels remain relatively stable, thanks to glycolytic compensation.
- While Complex I activity is maintained through compensatory mechanisms, Complexes III and IV show substantial decreases, impairing overall electron transport efficiency.
- The model reproduces mitochondrial swelling due to osmotic imbalance and ionic stress, leading to irreversible changes in the proton motive force (PMF), a form of stored energy across the mitochondrial membrane, similar to a battery’s voltage.
- SDH-b loss causes fumarate levels to drop and malate to accumulate, redirecting carbon flow through the malate/aspartate shuttle. This shift lowers mitochondrial NADH levels and disrupts oxidative phosphorylation, ultimately reinforcing the pseudohypoxic state observed in SDH-b–deficient cells.
Together, these alterations reinforce the pseudohypoxic state and set the stage for understanding how chromaffin cells maintain viability under stress.
No new biological discoveries were made in this study, but we were able to suggest a mechanistic link between SDH-b loss and cellular bioenergetic adaptation, i.e. the transition to a pseudo-hypoxic state despite oxygen availability in SDH deficient tumours.Furthermore, we parameterised the model with extensive 13C-labelling metabolic flux data from wild-type and SDH-b knockout chromaffin cells, increasing the mechanistic credibility of the model.— Ielyaas Cloete (CRM)
Mechanisms of Resilience and Therapeutic Potential
Importantly, the model helps explain how chromaffin cells retain Complex I activity under these conditions. This retention appears to be linked to cofactor oxidation, which contributes to managing mitochondrial swelling and limits the reversal of ATP synthase—allowing the cell to maintain viability without undergoing lysis. By capturing these key adaptive responses—such as Complex I compensation, ATP synthase reversal, and the shift toward a pseudohypoxic phenotype—the model also establishes itself as a versatile platform for investigating SDH-related dysfunctions in pheochromocytomas and other disease contexts.
Moreover, these findings offer a mechanistic explanation for the metabolic resilience of chromaffin cells in the face of SDH-b dysfunction and highlight potential targets—such as proton leak regulation and Complex I stabilization—for future therapeutic exploration.
A Platform for Future Exploration
While the current model simplifies certain aspects of cellular metabolism—such as treating glycolysis as a single net reaction or assuming a static extracellular environment—these choices have enabled a focused and computationally efficient framework centered on mitochondrial dynamics. Far from limiting its utility, they provide a solid foundation for future refinements.
Next steps include incorporating calcium fluxes, mitochondrial permeability transition pore (mPTP) behavior, and compartment-specific mitochondrial responses. These additions will enhance the model’s predictive power and biomedical relevance, allowing researchers to explore the interplay between metabolism and cellular stress signaling in greater detail.
For this study the bulk of my collaboration was with Elías. My role in the modelling process was including the regulatory effects of calcium on metabolite dynamics without modelling calcium dynamically. That being said, I enjoy collaborating with experimental biologists and clinicians, and figuring out how to use mathematics to answer questions and gain insights in physiology.
— Ielyaas Cloete (CRM)
Mathematics as a Tool for Biomedical Insight
This study exemplifies the power of mathematical modeling in biomedical research. By simulating the complex interplay between metabolism, ion transport, and mitochondrial dynamics, the authors offer a new lens through which to understand cellular resilience. The model provides a first step toward a robust framework for studying SDH-b loss and its consequences—not only in chromaffin cells but potentially in other pathological contexts.
As the model evolves, it may help uncover new strategies for stabilizing mitochondrial function in disease and inspire future research at the intersection of mathematics, biology, and medicine. Ultimately, this research shows how mathematics can help us understand—and perhaps one day treat—the hidden vulnerabilities of our cells.
|
|
CRM CommNatalia Vallina
|
CRM Awards Its Prize at Exporecerca Jove for the Third Time
For the third year running, CRM visited Exporecerca Jove to award its prize to the student project with the strongest mathematical content. This edition, the jury selected two winners: Xavier Ortiz Quintana, who built a real-time 3D scanner using...
When Symmetry Breaks the Rules: From Askey–Wilson Polynomials to Functions
Researchers Tom Koornwinder (U. Amsterdam) and Marta Mazzocco (ICREA-UPC-CRM) published a paper in Indagationes Mathematicae exploring DAHA symmetries. Their work shows that these symmetries shift Askey–Wilson polynomials into a continuous functional setting,and...
Homotopy Theory Conference Brings Together Diverse Research Perspectives
The Centre de Recerca Matemàtica hosted 75 mathematicians from over 20 countries for the Homotopy Structures in Barcelona conference, held February 9-13, 2026. Fourteen invited speakers presented research spanning rational equivariant cohomology theories, isovariant...
Three ICM speakers headline the first CRM Faculty Colloquium
On 19 February 2026, the Centre de Recerca Matemàtica inaugurated its first CRM Faculty Colloquium, a new quarterly event designed to bring together the mathematical community around the research carried out by scientists affiliated with the Centre. The CRM auditorium...
Trivial matemàtiques 11F-2026
Rescuing Data from the Pandemic: A Method to Correct Healthcare Shocks
When COVID-19 lockdowns disrupted healthcare in 2020, insurance companies discarded their data; claims had dropped 15%, and patterns made no sense. A new paper in Insurance: Mathematics and Economics shows how to rescue that information by...
L’exposició “Figures Visibles” s’inaugura a la FME-UPC
L'exposició "Figures Visibles", produïda pel CRM, s'ha inaugurat avui al vestíbul de la Facultat de Matemàtiques i Estadística (FME) de la UPC coincidint amb el Dia Internacional de la Nena i la Dona en la Ciència. La mostra recull la trajectòria...
Xavier Tolsa rep el Premi Ciutat de Barcelona per un resultat clau en matemàtica fonamental
L’investigador Xavier Tolsa (ICREA–UAB–CRM) ha estat guardonat amb el Premi Ciutat de Barcelona 2025 en la categoria de Ciències Fonamentals i Matemàtiques, un reconeixement que atorga l’Ajuntament de Barcelona i que enguany arriba a la seva 76a edició. L’acte de...
Axel Masó Returns to CRM as a Postdoctoral Researcher
Axel Masó returns to CRM as a postdoctoral researcher after a two-year stint at the Knowledge Transfer Unit. He joins the Mathematical Biology research group and KTU to work on the Neuromunt project, an interdisciplinary initiative that studies...
The 4th Barcelona Weekend on Operator Algebras: Open Problems, New Results, and Community
The 4th Barcelona Weekend on Operator Algebras, held at the CRM on January 30–31, 2026, brought together experts to discuss recent advances and open problems in the field.The event strengthened the exchange of ideas within the community and reinforced the CRM’s role...
From Phase Separation to Chromosome Architecture: Ander Movilla Joins CRM as Beatriu de Pinós Fellow
Ander Movilla has joined CRM as a Beatriu de Pinós postdoctoral fellow. Working with Tomás Alarcón, Movilla will develop mathematical models that capture not just the static architecture of DNA but its dynamic behaviour; how chromosome contacts shift as chemical marks...
Criteris de priorització de les sol·licituds dels ajuts Joan Oró per a la contractació de personal investigador predoctoral en formació (FI) 2026
A continuació podeu consultar la publicació dels criteris de priorització de les sol·licituds dels ajuts Joan Oró per a la contractació de personal investigador predoctoral en formació (FI 2026), dirigits a les universitats públiques i privades del...